
News - Page 5
Publication about a new facial rosacea skin care product

October 25, 2022
Rosacea: a skin disease of the face that affects the quality of life of patients
Rosacea is a chronic and progressive dermatological disease that manifests as centrofacial erythema and tends to worsen over time. The two most common forms of rosacea (vascular and papulopustular) mainly affect women aged 30 to 50 years. Although it is more easily diagnosed in patients with fair skin, rosacea affects all skin types.
Read morePink October 2022

October 18, 2022
Pink October is an annual campaign to raise awareness of breast cancer. Founded in 1985 by the American Cancer Society and Imperial Chemical Industries in the United States, breast cancer awareness month is now a worldwide event.
Read moreWorld Sight Day 2022

October 13, 2022
Sight is one of our five senses, and it is hard to imagine what it would be like if we lost our vision. Yet this is what happens to patients with optic neuritis (ON), the most common manifestation of neuromyelitis optica (NMO) and NMO spectrum disorders (NMOSD) in adults, as well as of myelin oligodendrocyte glycoprotein antibody disorders (MOGAD).
Read moreThe UNESCO International Day for Universal Access to Information 2022

September 28, 2022
September 28 is the “International Day for Universal Access to Information” (IDUAI), an annual event first organized by UNESCO in 2015.
What steps have been taken to improve universal access to scientific and medical information?
From a medical point of view, the introduction of “lay summaries” has helped to make the key results of clinical trials more understandable for all. In June 2021, the European Commission published Good Lay Summary Practice recommendations to provide guidance to sponsors for the writing of these “plain language summaries“. Indeed, the publication of these summaries is now required according to the new European Directive on clinical trials, European Union Clinical Trials Regulation (EU-CTR) 536/2014, which entered into force on 31 January 2022 and which aims to increase the transparency of clinical trials and ensure better standards for patient safety.
Read more“Virades de l’espoir” 2022: Walks of hope in France to help in the fight against cystic fibrosis

September 23, 2022
In our news update a few months ago we told you about cystic fibrosis, a rare disease that is one of the areas of expertise of our experienced medical and scientific writing team. In particular, we have already helped our clients with a range of projects on this topic, including:
- Congress reports of the international North American cystic fibrosis research (NACFC) held in the US
- Writing a scientific article from a day of exchanges between clinicians and biologists
- Translation of management and follow-up recommendations
- Creation of referenced slideshows.
The “Virades de l’espoir”, walks of hope to help in the fight against cystic fibrosis organized by the association Vaincre la Mucoviscidose in France, provide a timely opportunity to focus on this disease. Similar events take place in many countries around the world, including the Great Strides™ walking challenges organized by the Cystic Fibrosis Trust in the UK and the Cystic Fibrosis Foundation in the US.
Read moreWorld Alzheimer’s Day 2022
September 21, 2022
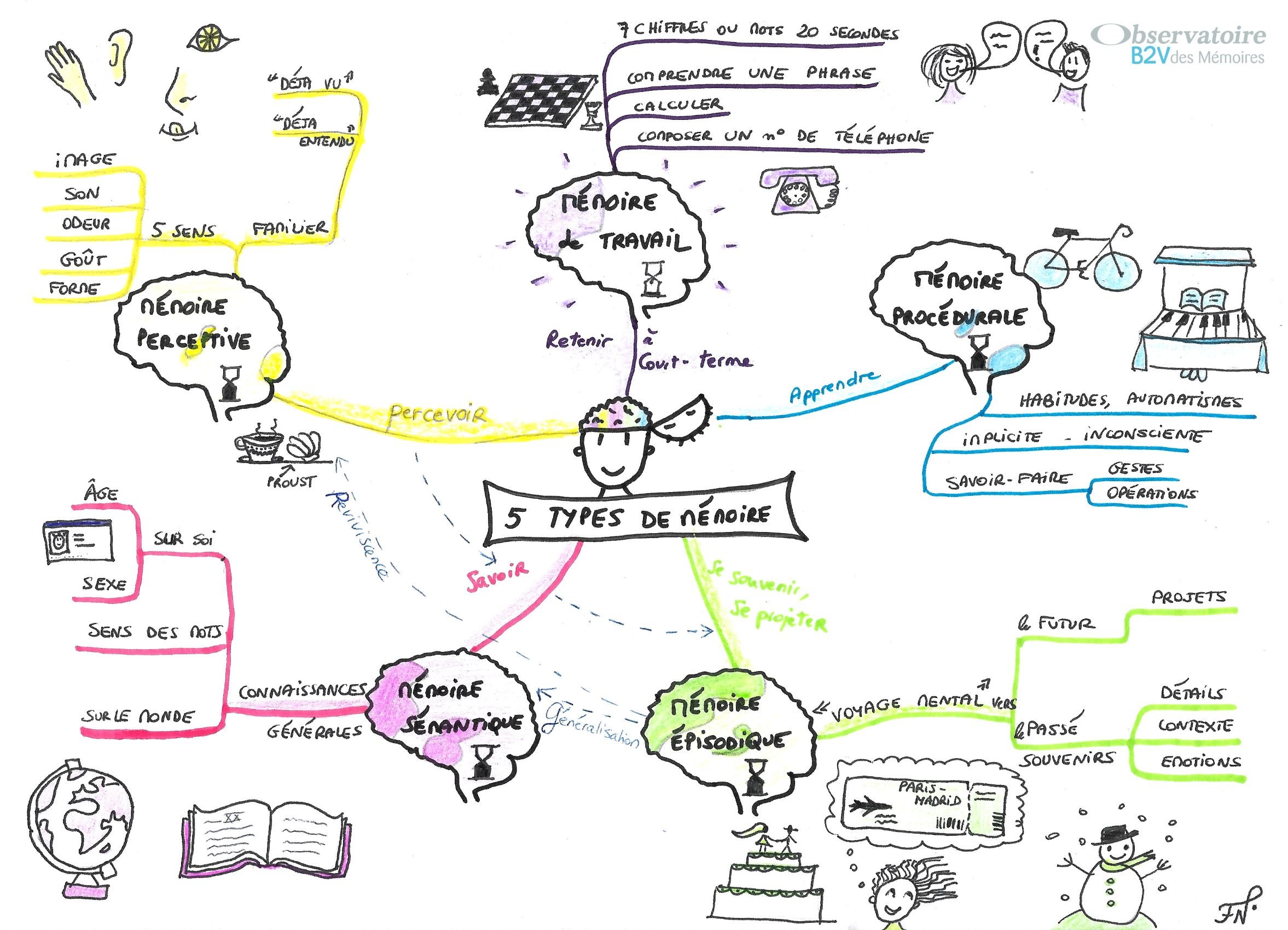
Alzheimer’s disease is a major global healthcare challenge. The number of people living with Alzheimer’s disease or other forms of dementia is currently estimated at over 55 million worldwide, and this number is expected to reach 78 million by 2030. The prevalence of dementia is estimated at around 10 million across all European countries, and is expected to almost double by 2050. In France, 2.2 million people may be affected by the disease by 2050.
Associations and foundations around the world, including Alzheimer’s disease international, the UK Alzheimer’s society, and the French Fondation Vaincre Alzheimer and the France Alzheimer association, organize events every year to raise awareness about the disease, and fight prejudice against those living with Alzheimer’s by highlighting the preserved abilities of those affected by the disease and encouraging the general public to see the disease in a new light.
This year, World Alzheimer’s Day is on September 21, 2022. As part of the theme, “Know dementia, Know Alzheimer’s”, we invite you to take another look at the impact of this neurodegenerative disease on the different types of memory: not all types of memory are affected in the same way by the disease, and some memories are relatively well preserved.
Read moreWorld Patient Safety Day 2022

September 13, 2022
Synergy Pharm keeps you informed about medication safety, the theme chosen by the World Health Organization (WHO) for the 2022 edition of World Patient Safety Day.
Read moreNew supplement dedicated to skin cancer

July 21, 2022
Synergy Pharm USA can support you from A to Z with the publication of your peer-reviewed journal supplement. We can produce your supplement from conference presentations or your study results. At our health communication agency, several scientific or medical articles can be written in parallel by our medical writers, which allows the rapid publication of the supplement after the event. Our editorial assistance and medical writing team can help to coordinate your project, reducing the delays associated with author proofreading, article review, submission and publication.
Read moreImplementation of the IVDR: Regulation (EU) 2017/746 for in vitro diagnostic medical devices
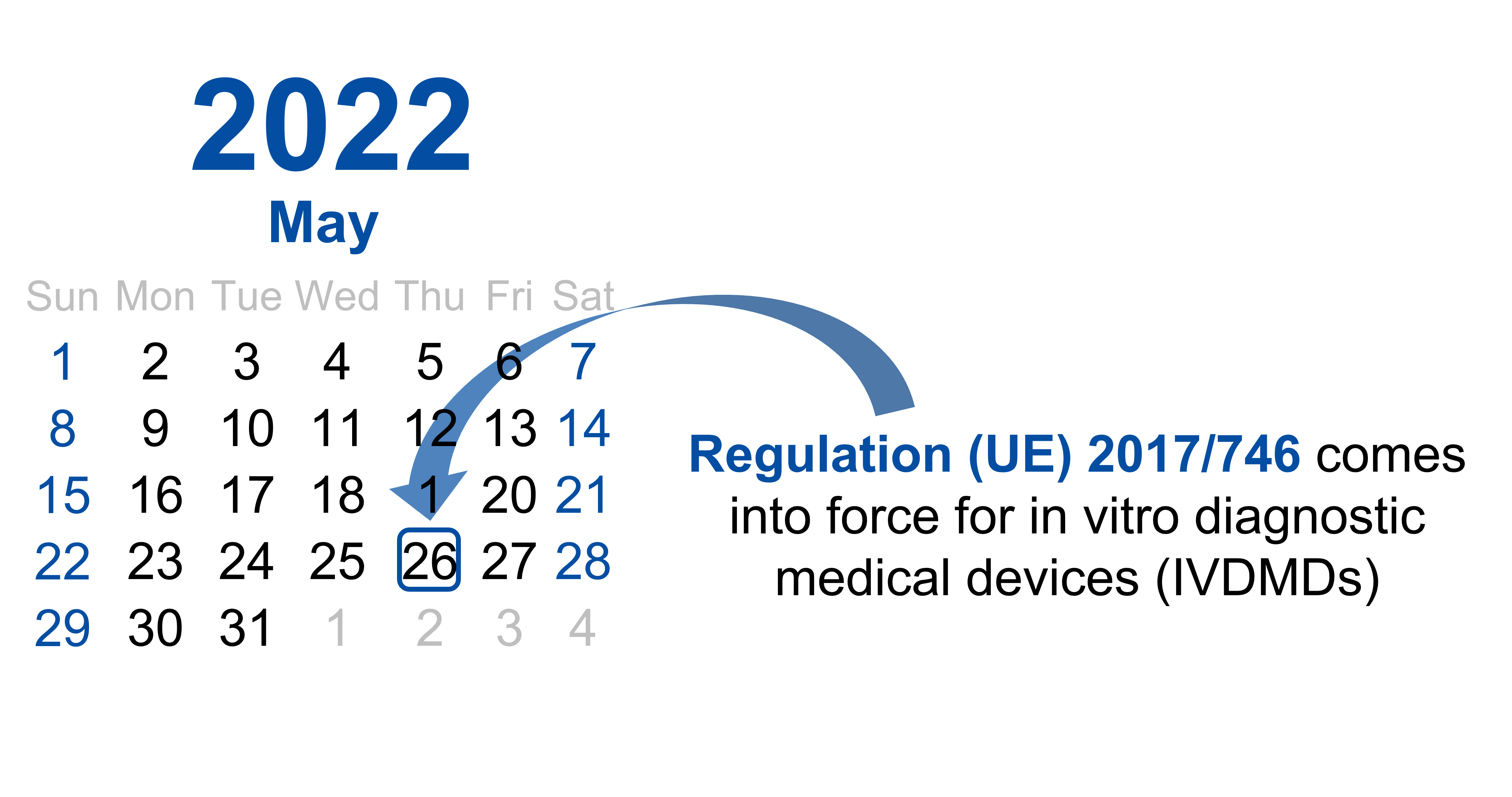
May 19, 2022
On 26 May 2022, Regulation (EU) 2017/746 will come into force for in vitro diagnostic medical devices (IVDMDs). Full details of this new regulation (IVDR) are available at: https://eur-lex.europa.eu/eli/reg/2017/746/oj
Read moreEuropean Patients’ Rights Day

April 18, 2022
April 18 is European Patients’ Rights Day (EPRD).
The European Charter of Patients’ Rights proclaims 14 rights that aim to guarantee “a high level of protection of human health” and to ensure the provision of high quality of health services in European countries, in particular during the Covid pandemic.
Read more